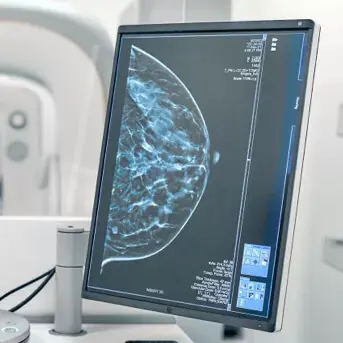
ScreenPoint Medical is showcasing the industry-leading Transpara Breast AI suite, an integrated set of solutions that includes Detection, Density, and Temporal Comparison at the 111th Annual Radiological Society of North America (RSNA) Annual Meeting, November 30-December 4, 2025. The most clinically validated Breast AI on the market, Transpara Breast AI is built for enterprise workflows to provide radiologists with a 'second pair' of eyes to help detect cancer earlier, reduce radiologist workload, and improve program performance.
2025 has been a pivotal year for ScreenPoint Medical. Surpassing 11 million mammograms processed, Transpara Breast AI is deployed in over 30 countries at leading healthcare providers and has been selected by 40 per cent of the 2025 US News and World Report's Top 20 Best Hospitals Honour Roll recipients.
Additionally, Transpara Breast AI has been chosen to be part of the only randomised controlled trials (RCTs) in the field of Breast AI: the MASAI RCT in Sweden and the upcoming $16mil PRISM RCT in the United States, which will commence in early 2026 and is spearheaded by researchers at UCLA and UC Davis. Results from MASAI, published earlier this year, found a 29 per cent increase in cancer detection and a 44 per cent decrease in screen-reading workload when using Transpara Detection compared to the standard of care.
Risk assessment is also a key theme at RSNA 2025: Transpara Risk, an image-based 5-year risk model for breast cancer, and Transpara Detection are the subjects of a powerful new study, "Added Value of Breast Cancer Risk Prediction Versus Detection Over Two Years with Mammography". This research shows the added value of risk prediction for precision care, with Transpara solutions outperforming both RSNA CAD and Mirai. AI-based risk analysis supports ScreenPoint Medical's commitment to women worldwide by powering personalised prevention pathways and enabling earlier diagnosis for less invasive treatment. Transpara Risk is approved for investigational use only.